Repetitive transcranial magnetic stimulation for alcohol use disorder //
This study is designed to test whether a non-invasive form of brain stimulation called repetitive transcranial magnetic stimulation (rTMS) affects negative emotions and alcohol use. Negative emotions include feelings such as anxiety, sadness, fear, anger, guilt and shame, irritability, and other unpleasant emotions.
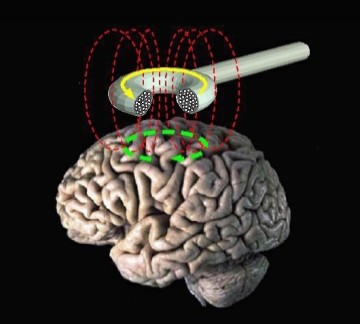 Transcranial magnetic stimulation is a magnetic pulse that can change the way your brain works for a short period of time. Repetitive TMS (rTMS) is a technique in which multiple magnetic pulses are administered over a short period of time. rTMS is approved by the FDA for the treatment of treatment-resistant depression and migraines. Early research has shown that rTMS applied to the cerebellum may help reduce feelings such as depression and anxiety, which might also help people reduce their drinking.
Transcranial magnetic stimulation is a magnetic pulse that can change the way your brain works for a short period of time. Repetitive TMS (rTMS) is a technique in which multiple magnetic pulses are administered over a short period of time. rTMS is approved by the FDA for the treatment of treatment-resistant depression and migraines. Early research has shown that rTMS applied to the cerebellum may help reduce feelings such as depression and anxiety, which might also help people reduce their drinking.
For this study, we are seeking individuals aged 22-65 who have an alcohol use disorder and are interested in cutting down or quitting drinking. Participants must be either receiving treatment for their alcohol use or willing to receive treatment for their alcohol use. The study will take place over 15 visits at MRN. Study participants will perform some paper and pencil and computerized tests, have two brain scans, and will also have 10 rTMS treatments over a two-week period. The study will also ask you some additional questions after the treatment has ended so that we can test whether the treatment worked. Because of this, participating in the study may take up to eight weeks in total. Participants are compensated up to $540 for the time and inconvenience involved with being in the study.
This study does involve in-person visits to our facility. Though safety procedures are put into place, there is still a risk of contracting COVID-19 when humans interact with one another. For this reason, you can choose to revoke your consent to participate in the study without penalty, for any reason, including if you feel unsafe at any time throughout the course of the study.

