
Traumatic Brain Injury //
Traumatic Brain Injury Research at MRN
It is undeniable that there has been a sea change regarding the potential long-term consequences of mild traumatic brain injury (mTBI), also referred to as concussion. As a result of a lack of observable pathology on CT, T1- or T2-weighted magnetic resonance imaging (MRI) findings (Hughes et al., 2004; Iverson, 2006), it was initially believed that mTBI resulted in limited behavioral and no long-term neurological consequences (Pellman et al., 2004). Large scale clinical studies in college athletes indicated the remittance of neurobehavioral symptoms typically within 7-10 days post-injury (McCrea et al., 2003) 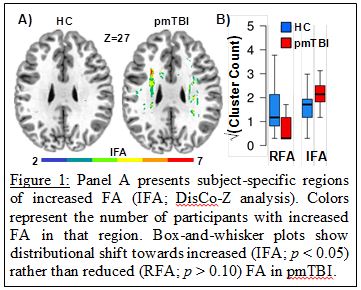

Published data from Dr. Mayer’s lab provides preliminary evidence of tissue-specific dysfunction and self-reported neuropsychiatric disturbances in the first few weeks of injury. This includes increased fractional anisotropy in white matter (see Figure 1), likely secondary to1) cytotoxic edema, 2) secondary inflammatory processes and/or 3) potential structural alterations in neurofilaments and myelin (Ling et al., 2012; Mayer et al., 2010; Mayer et al., 2012a). White matter creatine and the combined glutamate/glutamine peak (Glx) are also increased, possibly indicative of increased energetics for cell repair (Gasparovic et al., 2009; Yeo et al., 2011). Grey matter is characterized by reduced Glx, potentially representing an adaptive response to avoid excitotoxicity (Gasparovic et al., 2009; Yeo et al., 2011). A tight coupling exists between the release of glutamate into the synaptic cleft, the cycling of glutamate and glutamine between neurons and astrocytes, and the hemodynamic response. Therefore, it is no surprise that mTBI was also associated with hypoactivation of the reorienting network (see Figure 2) during spatial orienting (Mayer et al., 2009; Yang et al., 2012), hypoactivation within the dMFC and lateral PFC during multisensory selective attention (Mayer et al., 2012b), and neural abnormalities within sensory cortex (Mayer et al., 2015c). The balance of intrinsic neuronal fluctuations also appears to be affected by injury (see Figure 2), with reduced connectivity in the DMN and increased connectivity in frontoparietal attention networks (Mayer et al., 2011). Thus, our current working hypothesis is that a tissue- specific pattern of injury characterizes semi- acute mTBI, and that these underlying neuronal changes contribute to attentional dysfunction. A key goal of our future work is to determine which of these changes are most deleterious to cognition.
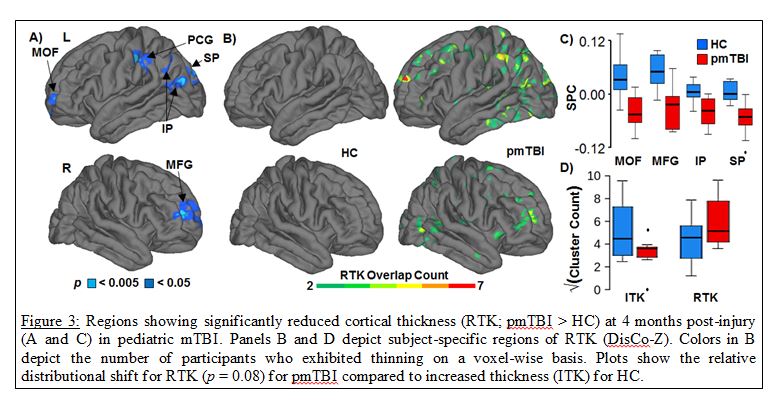
Mild TBI also presents an ideal “natural experiment” for examining both the initial consequences of the injury and the substrate for cognitive recovery (usually 3-6 months post-injury). Results from our lab and others suggest only a partial recovery in white matter (Ling et al., 2012; Mayer et al., 2010; Mayer et al., 2012a), with a more robust recovery of biochemical markers (Vagnozzi et al., 2010; Yeo et al., 2011). We also observed that biochemical recovery is impacted by general cognitive status (Yeo et al., 2011), representing an intriguing possibility about the role of cognitive reserve in mTBI. Reports of persistent hemodynamic and white matter abnormalities in clinically asymptomatic mTBI patients (Mayer et al., 2011; McAllister et al., 2006) may explain why a second, temporally proximal mTBI results in greater cognitive deficits than a single injury of comparable severity (Vagnozzi et al., 2005; Vagnozzi et al., 2008; Wall et al., 2006). A very recent finding (Mayer et al., 2015a) also suggests that pediatric mTBI is associated with decreases in cortical thickness at 4 months post-injury (see Figure 3), a finding absent in our adult patients (Ling et al., 2013).
We have also recently published several articles looking at the effects of repetitive mTBI in amateur and professional athletes, observing decreased cerebral blood flow (Meier et al., 2015) as well as structural and neurochemical abnormalities (Mayer et al., 2015b) at baseline and immediately post-injury relative to non-contact athletes. A more long-term goal (3-5 years) is to conduct longitudinal imaging studies on the relatively small percentage of patients with “complicated” mTBI and patients who have a poor outcome. Patients with complicated mTBI have visible focal lesions on CT scans and are more likely to have chronic neuropsychiatric symptoms and poor outcome following injury (Borgaro et al., 2003; Kashluba et al., 2008; Lange et al., 2009; Lee et al., 2008). Current theories (Bigler, 2010; Smits et al., 2008) suggest the focal lesions cause residual cognitive deficits. While intuitive, support for this theory has been mixed (Hughes et al., 2004; Lee et al., 2008; Smits et al., 2008). An alternative theory supported by our work is that poor cognitive outcomes are the result of diffuse injuries in otherwise healthy appearing tissue.
Published Articles for Research Participants in “Multimodal Imaging of Neuronal Injury” (HRRC #07-272)
Research //
- Mild Traumatic Brain Injury (mTBI) >
- Consequences of mTBI >
- Multi-Modal Imaging of Traumatic Brain Injury and Medical Cannabis >
- Reward-Guided Decision Making in Healthy Participants and Brain Injured Patients >
- The Impact of Diffuse Mild Brain Injury on Clinical Outcomes in Children >
Mild Traumatic Brain Injury (mTBI)
Mild traumatic brain injury (mTBI) is associated with neurobehavioral deficits in a majority of patients during the semi-acute injury phase, with a minority of patients remaining symptomatic for months to years post-injury. Routine clinical imaging scans (MRI and CT) are usually negative, suggesting that alternative neuroimaging techniques such as fMRI, DTI and 1H-MRS are well-positioned to provide unique information about the putative “silent lesions” of mTBI and their impact on neurobehavioral functioning. To this end, published data from my lab provides preliminary evidence of tissue-specific dysfunction and self-reported neuropsychiatric disturbances in both children and adults following mTBI. These injuries include increased fractional anisotropy in white matter, likely resulting from cytotoxic edema, secondary inflammatory processes, and potential structural alterations in neurofilaments and myelin. The brain’s ability to respond to external stimuli also appears to be reduced in the semi-acute stage of mTBI, with individual brain networks failing to communicate properly with each other. An increased understanding of the mechanisms underlying these injuries and how/when they recover represents the crucial next step for determining when patients can safely resume physical activities.
Consequences of mTBI
Almost all of us will get “hit in the head” at some point in our lives. “How hard” we get hit will likely determine whether we have a life altering experience or just walk away from it. However, emerging evidence suggests that even mild TBI can have prolonged consequences, affecting how we perform at work and make critical daily decisions for months. More importantly, particularly for athletes or soldiers, repeat injuries may make us more vulnerable for experiencing long-term negative outcomes. Unfortunately, mild TBI is not detected using routine clinical brain imaging techniques, nor do we have sufficient understanding of its long-term effects on behavior.
In the Mild TBI Project at MRN, lead by Dr. Andrew Mayer, we are investigating the subtle structural and biochemical consequences of mild TBI using state-of-the-art neuroimaging techniques. Our preliminary findings have shown that mild TBI causes alterations in the brain’s structure, function and chemistry. We are exploring how these alterations correlate with neurobehavioral symptoms, and how these may change as a function of recovery.
Multi-Modal Imaging of Traumatic Brain Injury and Medical Cannabis
Medical Cannabis has gained recognition for the management of a variety of conditions associated with traumatic brain injury due to its recent legal status in many states. However, there have not been any empirical studies exploring the beneficial and adverse effects of medical cannabis in persons with TBI using an extensive clinical and cognitive battery or multimodal neuroimaging before. This pilot study represents the first step in reducing the knowledge gap caused by the lack of empirical evidence supporting the use of medical cannabis for TBI, as it will serve as a guide for which measures of therapeutic targets and adverse outcomes should be included in future randomized control trials. At the end of this study, it is expected that the medical community will, for the first time, have preliminary data about the potential safety profile of using medical cannabis to treat neuropsychiatric symptoms following TBI, and whether the safety profile may vary according to the ratio of CBD to THC.
Reward-Guided Decision Making in Healthy Participants and Brain Injured Patients
The purpose of this project is to characterize the brain mechanisms underlying maladaptive reward processing in patients with mild traumatic brain injury (mTBI), with a focus on psychiatric symptoms in patients. Such a reward processing approach has seldom been adopted in the investigation of psychiatric symptoms in mTBI. The current protocol will involve extensive testing of cognitive and affective abilities in acute mTBI patients greater than 3 months post-injury – when psychiatric sequelae are particularly pronounced. An age- and education-matched sample of neurologically-healthy participants will also complete the study. The study will use a combination of questionnaires/ self-report assessment, behavioral assessments, and both structural (MPRAGE, DTI), and functional (resting-state fMRI, task fMRI) methodologies.
The Impact of Diffuse Mild Brain Injury on Clinical Outcomes in Children
There has been a lack of methodologically sound neuroimaging studies that have examined the diagnostic utility and predictive validity of more research-based neuroimaging techniques, such as fMRI and DTI, in traumatic brain injuries. This study aims to investigate the neuronal and behavioral correlates of attentional orienting and selective attention in a population of TBI patients post injury to determine if either aspect of attention is more affected by neuronal insult. With the use of fMRI, the project will investigate two aspects of attentional functioning (orienting and selective attention) in a well-characterized (neuropsychiatric testing) sample of patients with TBI during both the acute and recovery stages of injury.